Roots Analysis has announced the addition of “Genotoxicity / Mutagenicity Testing Services Market, 2023-2035” report to its list of offerings.
The report features an extensive study on the current landscape and the likely future potential of genotoxicity / mutagenicity testing services market over the next decade. The study also features an in-depth analysis, highlighting the capabilities of various stakeholders engaged in this domain. Amongst other elements, the report features:
- An executive summary of the insights captured during our research, offering a high-level view on the current state of the genotoxicity and mutagenicity testing service markets and its likely evolution in the short to mid and long term.
- A general overview of genotoxicity and mutagenicity, along with information on its detrimental effects, mechanism and testing techniques employed, key applications, recent developments in the market and future perspectives.
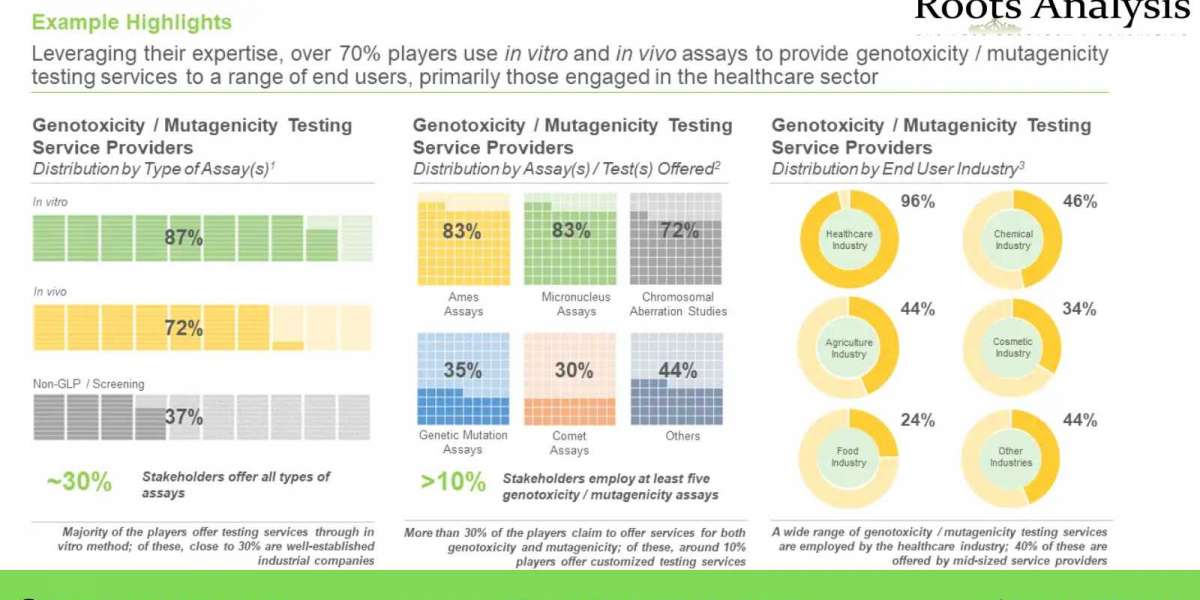
- A detailed assessment of the overall market landscape of genotoxicity and mutagenicity testing service providers, based on several relevant parameters, including year of establishment, company size (in terms of number of employees), location of headquarters, type of organization, location of the facility, type of operation (genotoxicity and mutagenicity), type of offering (service, and reagents and consumables), type of assay(s) (in vitro, in vivo and Non-GLP / Screening), assay(s) / test(s) offered (Ames test, micronucleus test, chromosomal aberration test, genetic mutation assay, comet assay and others), end user industry (healthcare industry, chemical industry, agriculture industry, cosmetic industry, food industry and other industries) and type of testing system(s) (bacteria, animals and novel technology)
- An insightful benchmark analysis of various service providers segregated into three peer groups, based on location of their headquarters (North America, Europe, and Asia-Pacific), highlighting the top players in this domain, in terms of their respective capabilities.
- Elaborate profiles of genotoxicity and mutagenicity service providers. Each profile includes a brief overview of the company, details related to its financial information (if available), service portfolio, recent developments, and an informed future outlook.
- An in-depth analysis of various publications related to genotoxicity and mutagenicity, based on several relevant parameters, such as year of publication, type of article, popular publishers (in terms of number of publications), popular journals (in terms of number of number of publications), journal impact factor and popular journals (in terms of journal impact factor). It also includes a publication timeline analysis (by article type and journal impact factor), along with benchmarking of publications to develop more insightful opinions on the recent trends related to research and development in this area.
- A detailed review of academic grants that have been awarded to various research institutes for projects focused on genotoxicity and mutagenicity, since 2018, based on several parameters, such as year of grant award, amount awarded, funding institute center, administering institute center, support period, purpose of grant, activity code, type of recipient organization, location of recipient organization, study section involved, type of grant application, popular NIH departments (in terms of number of grants), prominent program officers (in terms of number of grants) and popular recipient organizations (in terms of number of grants).
- A detailed analysis of recent partnerships inked between stakeholders engaged in this domain, since 2018, based on several relevant parameters, such as year of partnership, type of partnership, most active players (in terms of number of partnerships) and regional distribution of partnership activity in this domain.
- An in-depth analysis of various patents that have been filed / granted related to genotoxicity and mutagenicity, since 2018, taking into consideration parameters, such as type of patent, publication year, geographical region, CPC symbols, leading players (in terms of number of patents filled / granted) and type of applicant, along with a detailed patent benchmarking analysis and an insightful valuation analysis, highlighting the leading patents (in terms of number of citations).
- One of the key objectives of the report was to estimate the current opportunity and future growth potential of genotoxicity and mutagenicity testing services market over the coming years. We have provided informed estimates on the likely evolution of the market for the period, 2023-2035. Our year-wise projections of the current and future opportunity have further been segmented based on relevant parameters, such as type of assay, assay / test offered, end user industry and key geographical regions. In order to account for future uncertainties associated with some of the key parameters and to add robustness to our model, we have provided three market forecast scenarios, namely conservative, base, and optimistic scenarios, representing different tracks of the industry’s evolution.
By 2035, financial opportunity within the tissue engineering-based regeneration devices has been analyzed across the following segments:
- Type of Assay
- In vivo Assays
- In vitro Assays
- Non-GLP / Screening Assays
- Assay / Test Offered
- Comet Assays
- Micronucleus Assays
- Chromosomal Aberration Tests
- Genetic Mutation Tests
- Other Tests
- End User Industry
- Chemical Industry
- Healthcare Industry
- Agriculture Industry
- Cosmetic Industry
- Other Industries
- Geographical Regions
- North America
- Europe
- Asia-Pacific
- Latin America
- MENA
Key companies covered in the report
- Aurigene Pharmaceutical Services
- Charles River Laboratories
- GLR Laboratories
- Labcorp
- LSIM Safety Institute
- Sai Life Sciences
- Syngene
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/genotoxicity-testing-services-market.html
You may also be interested in the following titles:
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415